Iso 13485 2016 Standard Pdf
Looking for the text of ISO 13485:2016?
- This FAQ document is designed to answer some key questions around ISO and EN ISO. Questions are grouped by key theme. The document accompanies two BSI Webinars covering the scope of the new standard, and a discussion of both ISO and ISO 9001:2015. For more information, please see the ISO revision webpage.
- ISO 13485 certification is a valuable credential put in place to keep professionals and customers safe in clinics, hospitals and other medical settings. ISO is based on the ISO 9001 process model approach and is a management systems standard specifically developed for.
You will need to purchase a copy of the standard to reach certification (sometimes multiple standards are required). Due to copyright restrictions, we are not able to include these with our products. So, we have partnered with Techstreet, an authorized seller of ISO Standards. We have sorted each standard into Needed, Recommended, and Additional Related Standards. Each has a link to purchase through Techstreet.
BSI is committed to ensuring a smooth assessment for all clients wishing to certify to ISO, whether you are new to the standard or transitioning from ISO / EN ISO. This document allows you to detail how you intend to meet the additional requirements of the new standard, so should be used in conjunction with ISO.
Needed for ISO 13485 Certification:
ISO 13485:2016 –Medical devices – Quality management systems – Requirements for regulatory purposes

ISO 14971:2019– Medical devices – Application of risk management to medical devices
Recommended for ISO 13485 Certification:
- ISO Guide 73:2009 – Risk management – Vocabulary

Additional Related Standards:
- ISO 19011:2018 – Guidelines for Auditing Management Systems
Iso 13485 2016 Standard Free Pdf


Iso 13485 2016 Pdf Download
ISO 9001 Family
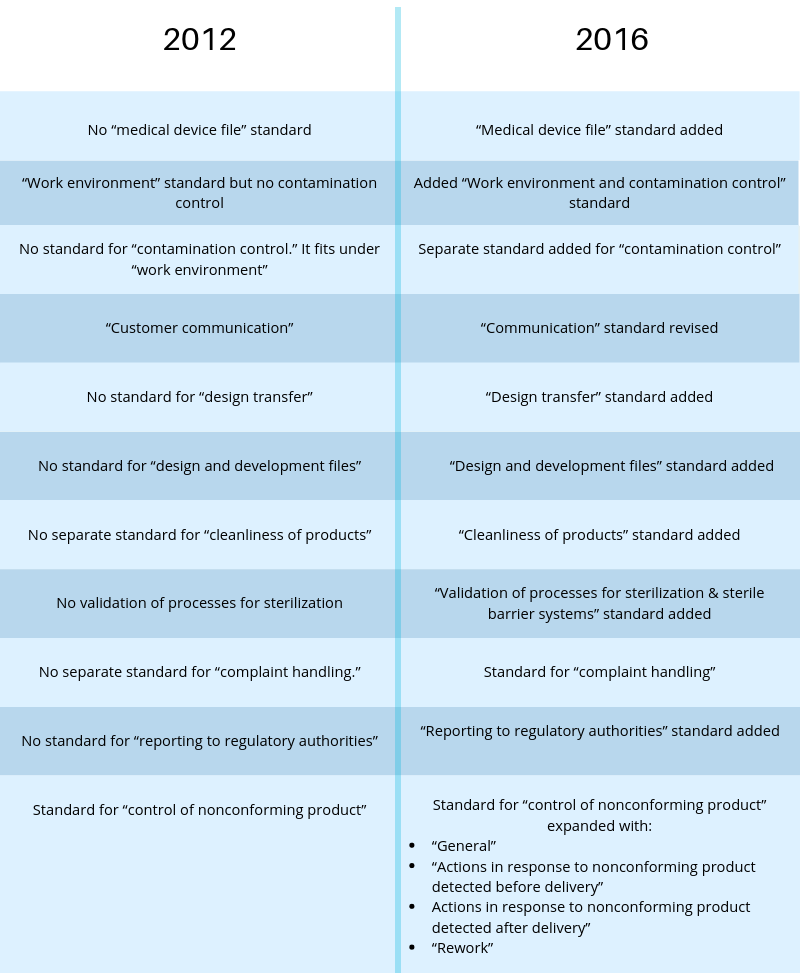
- ISO 9000:2015 – Quality Management Systems – Fundamentals and Vocabulary
- ISO 9001:2015 – Quality Management Systems – Requirements
- ISO 9002:2016 – Guidelines for the application of ISO 9001:2015
- ISO 9004:2018 – Quality Management – Quality of an organization – Guidance to achieve sustained success
Medical Device Software
- IEC 62304 Ed. 1.1 b:2015 – Medical device software – Software life cycle processes – Consolidated Edition
- IEC/TR 80002-1 2009 – Medical device software – Part 1: Guidance on the application of ISO 14971 to medical device software
- ISO/TR 80002-2:2017 – Medical device software – Part 2: Validation of software for medical device quality systems
- ISO 31000:2018 – Risk Management Guidelines
These standards are sold by the Techstreet website, a reseller of ISO Standards that includes ISO, SAE, IATF, and other standards. Many standards are available to download in pdf format. Purchase transactions are conducted on Techstreet’s secure site and are not combined with a purchase from 13485Store.
Iso 13485 Pdf Free Download
